Ljudje would not have existed without virusi because viral protein plays a key role in the development of človeškega embryo. However, at times, they pose existential threats in the form of diseases as in the case of current COVID-19 pandemic. Ironically, virusi comprise ~8% of our genome, that has been acquired during the course of evolution, making us “virtually a chimera”.
Najbolj razvpita in grozljiva beseda leta 2020 je brez dvoma 'virus'. Roman koronavirus je odgovoren za trenutno bolezen COVID-19 brez primere in skorajda propad svetovnega gospodarstva. Vse to povzroča droben delček, ki se niti ne šteje za 'popolnoma' živega, ker je zunaj gostitelja v nefunkcionalnem stanju, v notranjosti pa se ohranja šele ob okužbi gostitelja. Bolj presenetljivo in šokantno je dejstvo, da je ljudje have been carrying the viral “genes” since times immemorial and currently viral genes constitutes ~8% of the človeškega genome (1). Just to put this in perspective, only ~1% človeškega genome is functionally active responsible for making proteins that determine who we are.
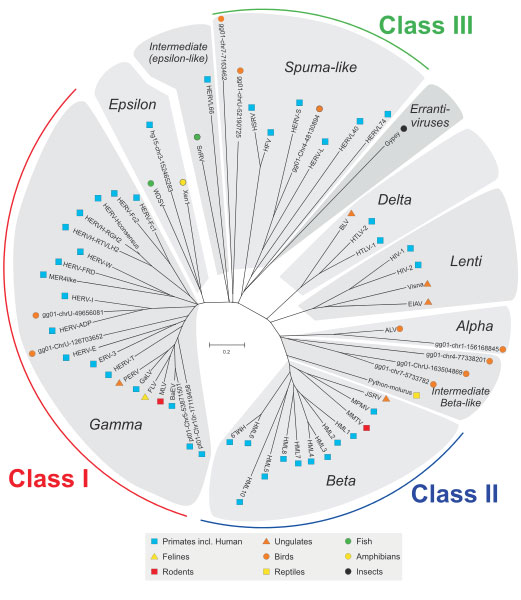
The story of relationship between ljudje in virusi started 20-100 million years ago when our ancestors got infected by virusi. Each endogenous retrovirus family is derived from a single infection of the germline cells by an exogenous retrovirus that after integrating into our ancestor, expanded and evolved (2). The propagation followed by the horizontal transfer from parents to offspring and today we have these viral genomes embedded in our DNA as človeškega endogenous retroviruses (HERVs). This is a continuous process and may even be happening at the moment. Over the course of evolution, these HERVs acquired mutations, became stabilised in the človeškega genome and lost their ability to cause the disease. The endogenous retrovirusi are not only present in ljudje but are omnipresent in all living organisms. All these endogenous retroviruses grouped into three classes (Class I, II and III) occurring across different animal species exhibit a phylogenetic relationship based on their sequence similarity (3) as depicted in Figure below. HERVs belong to the Class I group.
Of the various embedded retroviruses present in the človeškega genome, a classic example worth mentioning here, is that of a retroviral protein that is highly fusogenic envelope protein called syncytin, (5) whose original function in the virus was to fuse with host cells to cause infection. This protein has now been adapted in ljudje to form placenta (fusion of cells to make multinucleated cells) that not only provides food to foetus from the mother during pregnancy but also protects the foetus from the mother’s immune system due to the immunosuppressive nature of the syncytin protein. This particular HERV has proven to be beneficial to the človeškega race by defining its very existence.
HERVs have also been implicated in providing innate immunity to the host by preventing further infection from related virusi or reducing the severity of the disease upon re-infection by similar type of virusi. A 2016 review by Katzourakis and Aswad (6) describes that endogenous virusi can act as regulatory elements for genes that control immune function, thereby leading to immunity development. In the same year, Chuong et al (7) demonstrated that certain HERVs act as regulatory enhancers by modulating the expression of IFN (interferon) inducible genes thereby providing innate immunity. HERV expression products can also act as pathogen-associated molecular patterns (PAMPs), triggering the cellular receptors responsible for host first line of defences (8-10).
Še en zanimiv vidik HERV-jev je, da nekateri od njih kažejo insercijski polimorfizem, to pomeni, da je v genomu zaradi insercijskih dogodkov prisotno različno število kopij. Študija 20 oseb, ki pripadajo različnim etničnim skupinam, je pokazala vzorce polimorfizma vstavljanja med 0-87 % pri vseh preiskovancih (11). To lahko vpliva na povzročanje bolezni z aktivacijo določenih genov, ki so sicer tihi.
Dokazano je tudi, da so nekateri HERV povezani z razvojem avtoimunskih motenj, kot je multipla skleroza (12). V normalnih fizioloških pogojih je ekspresija HERV strogo regulirana, medtem ko lahko pri patoloških stanjih zaradi sprememb v zunanjem/notranjem okolju hormonske spremembe in/ali mikrobna interakcija povzročijo disregulacijo izražanja HERV, kar vodi do bolezni.
The above characteristics of HERVs suggest that not only their presence in človeškega genome is inevitable but they possess the ability to regulate the homeostasis of the immune system either by activating or suppressing it, thereby causing differential effects (from being beneficial to causing a disease) in hosts.
The COVID-19 pandemic is also caused by a retrovirus SARS-nCoV-2, that belongs to the influenza family, and it may be plausible that, during the course of evolution, genomes related to this family of virusi got integrated into the človeškega genome and are now present as HERVs. It is surmised that these HERVs might exhibit different polymorphisms, as mentioned above, among people of different ethnicity. These polymorphisms may be in the form of differential copy number of these HERVs and/or presence or absence of mutations (changes in the genome sequence) accumulated over a period of time. This variability in the integrated HERVs may offer an explanation for the differential mortality rates and the severity of COVID-19 disease in different countries effected by the pandemic.
***
Reference:
1. Griffiths DJ 2001. Endogenous retroviruses in the človeškega genome sequence. Genome Biol. (2001); 2(6) Reviews 1017. DOI: https://doi.org/10.1186/gb-2001-2-6-reviews1017
2. Boeke, JD; Stoye, JP (1997). "Retrotranspozoni, endogeni retrovirusi in evolucija retroelementov". V Coffin, JM; Hughes, SH; Varmus, HE (ur.). Retrovirusi. Laboratorijska tisk Cold Spring Harbor. PMID 21433351.
3. Vargiu L, et al. Classification and characterization of človeškega endogenous retroviruses; mosaic forms are common. Retrovirology (2016); 13: 7. DOI: 10.1186 / s12977-015-0232-y
4. Classes_of_ERVs.jpg: Jern P, Sperber GO, Blomberg J (izpeljanka: Fgrammen (govor)), 2010. Dostopno na spletu na https://commons.wikimedia.org/wiki/File:Classes_of_ERVs.svg Dostop 07. maja 2020
5. Blond, JL; Lavillette, D; Cheynet, V; Bouton, O; Oriol, G; Chapel-Fernandes, S; Mandrandes, S; Mallet, F; Cosset, FL (7 April 2000). “An envelope glycoprotein of the človeškega endogenega retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor”. J. Virol. 74 (7): 3321–9. DOI: https://doi.org/10.1128/jvi.74.7.3321-3329.2000.
6. Katzourakis A, and Aswad A. Evolution: Endogenous Virusi Provide Shortcuts in Antiviral Immunity. Current Biology (2016). 26: R427-R429. http://dx.doi.org/10.1016/j.cub.2016.03.072
7. Chuong EB, Elde NC in Feschotte C. Regulativna evolucija prirojene imunosti s sodelovanjem endogenih retrovirusov. Znanost (2016) letnik. 351, številka 6277, str. 1083-1087. DOI: https://doi.org/10.1126/science.aad5497
8. Wolff F, Leisch M, Greil R, Risch A, Pleyer L. Dvorezen meč (ponovnega) izražanja genov s hipometilacijskimi sredstvi: od virusne mimikrije do izkoriščanja kot primarnih sredstev za ciljno modulacijo imunske kontrolne točke. Cell Commun Signal (2017) 15:13. DOI: https://doi.org/10.1186/s12964-017-0168-z
9. Hurst TP, Magiorkinis G. Activation of the innate immune response by endogenous retrovirusi. J Gen Virol. (2015) 96:1207–1218. DOI: https://doi.org/10.1099/vir.0.000017
10. Chiappinelli KB, Strissel PL, Desrichard A, Chan TA, Baylin SB, Correspondence S. Zaviranje metilacije DNA povzroči interferonski odziv pri raku preko dsRNA, vključno z endogenimi retrovirusi. Celica (2015) 162: 974–986. DOI: https://doi.org/10.1016/j.cell.2015.07.011
11. Mehrab G, Sibel Y, Kaniye S, Sevgi M and Nermin G. Human endogenous retrovirus-H insertion screening. Molecular Medicine Reports (2013). DOI: https://doi.org/10.3892/mmr.2013.1295
12. Gröger V in Cynis H. Humani endogeni retrovirusi in njihova domnevna vloga pri razvoju avtoimunskih motenj, kot je multipla skleroza. Sprednji mikrobiol. (2018); 9: 265. DOI: https://doi.org/10.3389/fmicb.2018.00265
***